MEDICAL RESEARCH
OUR FOCUS
Our mission is to find a cure for Facioscapulohumeral muscular dystrophy (FSHD). A disease that affects an estimated two million people globally. It is caused by an overexpression of a protein called DUX4, which is toxic to muscle.
The true prevalence of this disease is still unknown. Due to poor diagnostics and misdiagnosis, many people live unaware they carry the genetic gene, at risk of passing down generations.
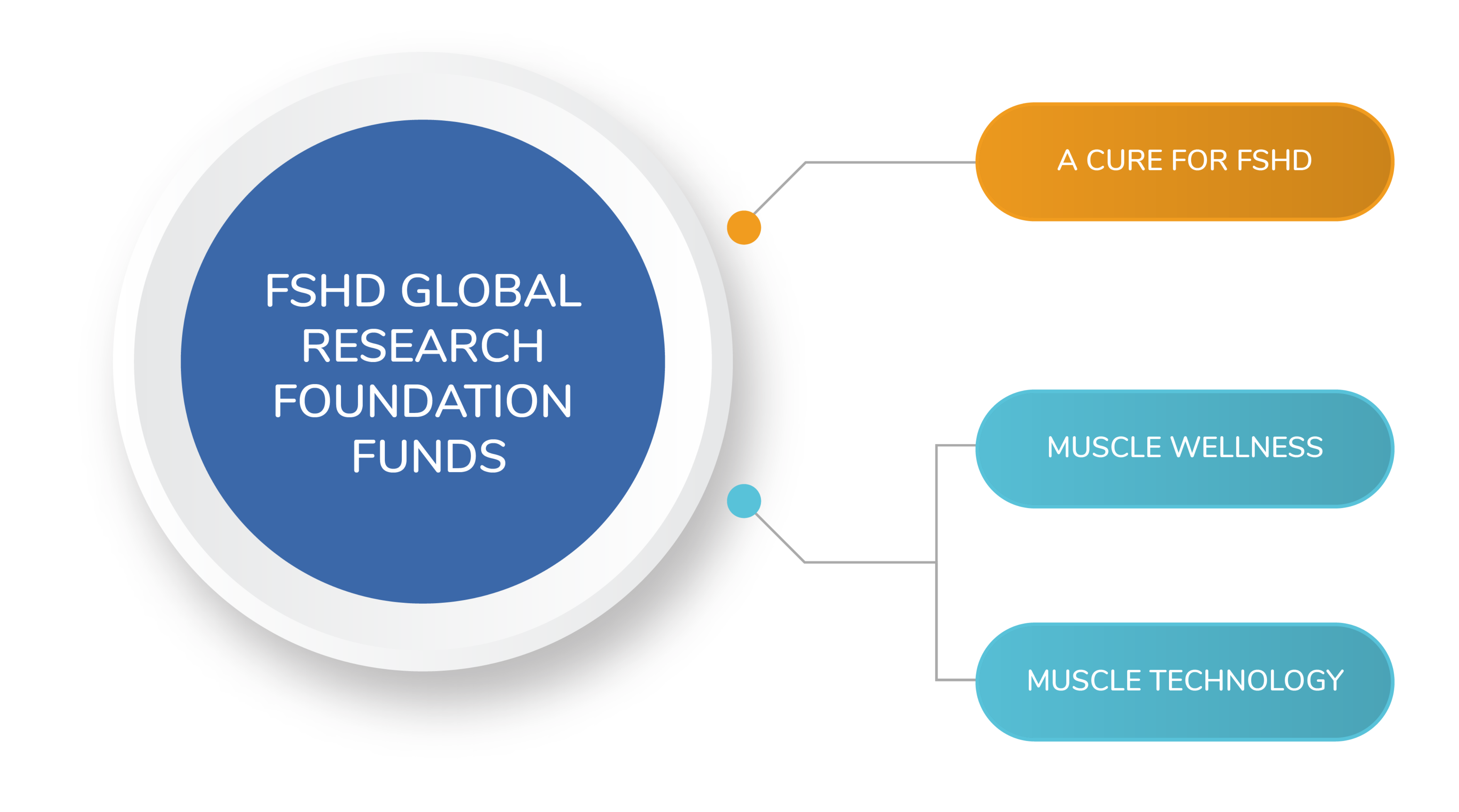
The Foundation undertakes a wide range of medical research focused on; slowing this disease, muscle wellness and muscle technology. The aim of this research is not only to find a cure for FSHD, but to find ways that all people suffering from muscle weakness caused by neuromuscular disorders, muscle trauma and ageing will benefit.
OUR FUNDING PILLARS
OUR JOURNEY TO A CURE
Our mission is to find a cure for Facioscapulohumeral muscular dystrophy (FSHD). A disease that affects an estimated two million people globally. It is caused by an overexpression of a protein called DUX4, which is toxic to muscle.
The true prevalence of this disease is still unknown. Due to poor diagnostics and misdiagnosis, many people live unaware they carry the genetic gene, at risk of passing down generations.
The Foundation undertakes a wide range of medical research focused on; slowing this disease, muscle wellness and muscle technology. The aim of this research is not only to find a cure for FSHD, but to find ways that all people suffering from muscle weakness caused by neuromuscular disorders, muscle trauma and ageing will benefit.